Introduction:
Why do scientists spend time looking for water on other planets? Why is water so important? It is because water is essential to life as we know it. Water is one of the more abundant molecules and the one most critical to life on Earth. Approximately 60–70 percent of the human body is made up of water. Without it, life as we know it simply would not exist.
The polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the processes of life. Life originally evolved in a watery environment, and most of an organism’s cellular chemistry and metabolism occur inside the watery contents of the cell’s cytoplasm. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions that leads to the generation of pH. Understanding these characteristics of water helps to elucidate its importance in maintaining life.
Structure and Polarity of Water
Access the worksheet here, and make a copy to your own Google Drive to complete it (you will not be able to edit the original document):
https://docs.google.com/document/d/1NiO6fqhx6bRcaGej63Le6KeQnLK4-ZN2SEd1-1_WrLE/edit?usp=sharing
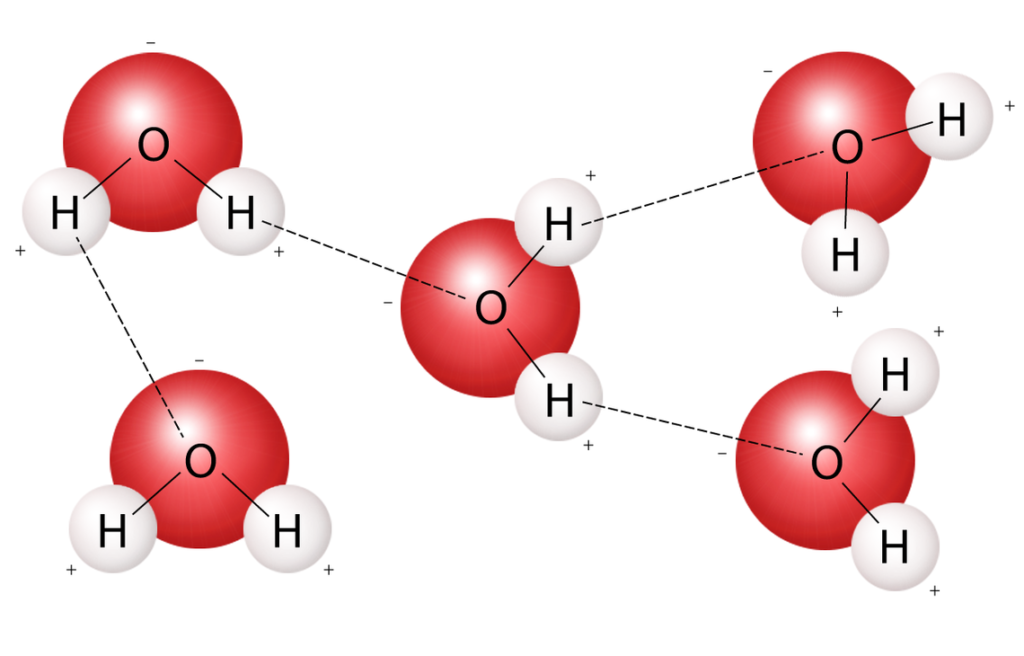
One of water’s important properties is that it is a polar molecule: the hydrogen and oxygen within water molecules (H2O) share electrons, forming what are known as covalent bonds. While there is no net charge to a water molecule, there is a slightly positive charge on the hydrogens and a slightly negative charge on the oxygen, contributing to water’s properties of attraction. Water’s charges are generated because oxygen has 8 electrons, making it more electronegative than hydrogens, with just 1 electron each. This makes it more likely that a shared electron would be found near the oxygen nucleus than the hydrogen nucleus, thus generating the partial negative charge near the oxygen.
As a result of water’s polarity, when two water molecules get close together, each water molecule attracts other water molecules because of the opposite charges. The oxygen atom of one molecule will bind with hydrogen atoms of the other molecule, forming hydrogen bonds. Hydrogen bonds are not as strong as covalent bonds, but they are strong enough to bind water molecules together and give water its unique characteristics. A good analogy is that covalent bonds are like a strong glue bond while the hydrogen bonds are like the bond between two small magnets.
In the worksheet, answer the following questions:
- What is a polar molecule?
- Name the elements that make up water:
- What charge do the hydrogen atoms have in a molecule of water?
- What charge does the oxygen atom have in a molecule of water?
- What type of bonds hold the oxygen and hydrogens together in a single water molecule?
- The __________________ atom of one water molecule will bond with ___________________ atoms of other water molecules.
- What type of bonds hold together multiple water molecules?
- Of the two bonds discussed today, which is the stronger? Which is weaker?
- On paper, or in an online drawing tool of your choice, draw one molecule of water and label the hydrogen and oxygen atom, and bonds. Label the positive (+) region of the molecule and the negative (-) region of the molecule.
- Draw 5 molecules of water bonded together with hydrogen bonds. Label the hydrogen bonds, covalent bonds, oxygen atoms, and hydrogen atoms.
Water is an Important Solvent
A polar substance that interacts readily with or dissolves in water is referred to as hydrophilic (hydro- = “water”; -philic = “loving”). In contrast, non-polar molecules such as oils and fats do not interact well with water, and separate from it rather than dissolve in it, as we see in salad dressings containing oil and vinegar (an acidic water solution). These nonpolar compounds are called hydrophobic (hydro- = “water”; -phobic = “fearing”).
Since water is a polar molecule with slightly positive and slightly negative charges, ions and polar molecules can readily dissolve in it. Therefore, water is referred to as a solvent, a substance capable of dissolving other polar molecules and ionic compounds. The charges associated with these molecules will form hydrogen bonds with water, surrounding the particle with water molecules. This is referred to as a hydration shell, and it serves to keep the particles separated or dispersed in the water.
Solutions and suspensions: Water is not always pure – it is often found as part of a mixture. A mixture is a material composed of two or more elements or compounds that are physically mixed together but not chemically combined. Salt and pepper stirred together constitute a mixture, as do sugar and sand. Earth’s atmosphere is a mixture of gases. Living things are in part composed of mixtures involving water. Two types of mixtures that can be made with water are solutions and suspensions.
If a crystal of table salt is placed in a glass of warm water, sodium and chloride ions on the surface of the crystal are attracted to the polar molecules. Ions break away from the crystal and are surrounded by water molecules. The ions gradually become dispersed in the water, forming a type of mixture called a solution. All the components of a solution are evenly distributed throughout the solution. In a salt-water solution, table salt is the solute, the substance that is dissolved. Water is the solvent – the substance in which the solute dissolves. Water’s polarity gives it the ability to dissolve both ionic compounds and other polar molecules, such as sugar.
A suspension is a mixture between two substances, one of which is finely divided and dispersed in the other. Common suspensions include oil in water, and dust in air. Particles in a suspension are larger than those in a solution and will settle out if the suspension stands undisturbed. Nonpolar substances may form suspensions in water.
Text adapted from “Biology” by Openstax College. Download for free at: http://cnx.org/content/col11448/latest/
Activity: Watch the “Solutions and Suspensions” video: (video does not have any narration; music only)
Alternately, if you would like to duplicate this experiment at home, you’ll need:
- Two containers for water (clear glass or plastic is best)
- Table salt
- Cooking oil
- Experimental Protocol:
- Fill the glasses with an equal amount of water (about 1 cup)
- Put a tablespoon of table salt in one of the glasses, and a tablespoon of oil in the other
- Stir each solution for one minute, and then describe the appearance and content of the contents of each beaker.
Answer the following questions:
- Which of the substances (salt or oil) formed a suspension?
- Which of the substances (salt or oil) formed a solution?
- With the properties of water and polarity in mind, come up with a hypothesis about why the salt behaved as it did when added to water.
Adhesion and Cohesion
A single water molecule may be involved in as many as four hydrogen bonds at the same time. The ability of water to form multiple hydrogen bonds is responsible for many of water’s properties. Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion, which is an attraction between molecules of the same substance. Because of hydrogen bonding, water is extremely cohesive, keeping the molecules together at the liquid-gas (water-air) interface, although there is no more room in the glass.
Cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This is why water forms droplets when placed on a dry surface rather than being flattened out by gravity. It’s even possible to “float” a needle on top of a glass of water if it is placed gently without breaking the surface tension.
Adhesion is the attraction between water molecules and other molecules. Adhesion is observed when dew drops cling to the strands of a spider’s web. You can also witness adhesion when water “climbs” up a glass tube that has been placed in a glass of water: the water appears to be higher on the sides of the tube than in the middle. This is because the water molecules are attracted to the charged glass walls of the capillary more than they are to each other and therefore adhere to it.
Why are cohesive and adhesive forces important for life? Cohesive and adhesive forces are important for the transport of water from the roots to the leaves in plants. These forces create a “pull” on the water column. This pull results from the tendency of water molecules being evaporated on the surface of the plant to stay connected to water molecules below them, and so they are pulled along. Plants use this natural phenomenon to help transport water from their roots to their leaves. Without these properties, plants would be unable to receive the water and the dissolved minerals they require. In another example, insects such as the water strider use the surface tension of water to stay afloat on the surface layer of water and even mate there
- How many hydrogen bonds can a single water molecule have?
- Define cohesion.
- Define adhesion.
To see a demonstration of cohesion and adhesion, watch the “Drops on a Penny” video. The video features three “trials,” and as you watch, you will count the following three times each (for a total of nine observations). Record your data in the chart in your worksheet.
- How many drops of water can fit on a penny before spilling over
- How many drops of oil can fit on a penny before spilling over
- How many drops of alcohol can fit on a penny before spilling over
(Video does not have any narration; music only)
4. On paper, or an online drawing tool, draw how the looks on the penny.
5. In the table with your data, calculate the average number of drops for each substance.
6. Based on your results, which of these three substances has the highest surface tension? Which has the least surface tension? Why?
7. What property of water allows the water to stick to the penny?
8. What property of water allows the water to form a dome-like structure on top of the penny?
9. Describe an example of cohesion and adhesion that you might observe during your daily life.
Properties of Ice
The formation of hydrogen bonds is an important quality of the liquid water that is crucial to life as we know it. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high water content, understanding these chemical features is key to understanding life.
In liquid water, hydrogen bonds are constantly formed and broken as the water molecules slide past each other. The breaking of these bonds is caused by the motion (kinetic energy) of the water molecules due to the heat contained in the system. When the heat is raised as water is boiled, the higher kinetic energy of the water molecules causes the hydrogen bonds to break completely and allows water molecules to escape into the air as gas (steam or water vapor).
On the other hand, when the temperature of water is reduced and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding (there is not enough energy to break the hydrogen bonds) that makes ice less dense than liquid water, a phenomenon not seen in the solidification of other liquids.
Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: the water molecules are pushed farther apart compared to liquid water. With most other liquids, solidification when the temperature drop includes the lowering of kinetic energy between molecules, allowing them to pack even more tightly than in liquid form and giving the solid a greater density than the liquid. The lower density of ice, an anomaly, causes it to float at the surface of liquid water, such as in an iceberg or in the ice cubes in a glass of ice water. In lakes and ponds, ice will form on the surface of the water creating an insulating barrier that protects the animals and plant life in the pond from freezing. Without this layer of insulating ice, plants and animals living in the pond would freeze in the solid block of ice and could not survive.
When sea ice forms, freshwater freezes and leaves behind a concentrated salt solution called brine. This brine is found in pockets throughout the ice. Brine pockets allow organisms that get trapped in the ice to avoid freezing and survive until the next spring. The pockets are small and isolated in winter, but in spring, as the ice begins to warm, the brine pockets get bigger and combine with other pockets to form channels which allow the organisms to move throughout the ice. The dark color at the bottom of these ice cores indicates the presence of algae. The detrimental effect of freezing on living organisms is caused by the expansion of ice relative to liquid water. The ice crystals that form upon freezing rupture the delicate membranes essential for the function of living cells, irreversibly damaging them. Cells can only survive freezing if the water in them is temporarily replaced by another liquid like glycerol.
1. Why can solid ice float on liquid water?
2. When a lake freezes, how does the density of water prevent the living organisms in the lake from freezing as well?
View the video “Salt and Fresh Water Ice,” to observe the behavior of dye on two types of ice. Pay attention to the following: How did the dye behave on the fresh water ice? On the salt water ice? (Video does not have any narration; music only)
Record your observations in the worksheet.
3. How would you explain your observations? Why did the dye behave differently on the two pieces of ice?
4. How can the saline content in ice help some aquatic organisms survive cold temperatures?
Surface Tension
Water has very high surface tension, which results from water molecules’ strong attraction toward each other. The molecules on the surface of the water and those below them are attracted to each other so tightly that a sort of “skin” forms on the surface. It is surface tension that allows some objects that are more dense than water to float on it, and insects are able to walk on water thanks to surface tension.
In a few minutes, you will watch a video, or conduct your own experiment, in which a boat is powered by using liquid dish soap. How does this work? The surface tension allows the cardboard boat to float on top of the water. By adding soap, the arrangement of the water molecules is disrupted. The water molecules near the detergent are attracted to the detergent as well as to other water molecules, so the surface tension of the water behind the boat decreases. Water molecules move from areas of low surface tension to areas of high surface tension. The boat is pulled towards areas of high surface tension by the water in front of the boat.
Text adapted from “Biology” by Openstax College. Download for free at: http://cnx.org/content/col11448/latest/
Watch the video, “Toothpick Star” (video does not have any narration; music only).
Alternately, if you have toothpicks at home, you can replicate this experiment on your own.
- Protocol:
- One at a time, with thumbs together at the middle of the toothpick, bend the toothpick until it breaks, but DO NOT BREAK COMPLETELY.
- Arrange all five toothpicks on a dry surface (a small plate works well)
- Now, drop 3-4 drops of water at the center of the arrangement so that the water touches the breaks in the toothpicks. Observe what happens.
1. Describe the behavior of the toothpicks.
2. Provide an explanation for the behavior you observed, based on the properties of water we’ve explored today.
Now, we’re going to demonstrate these properties of water by disrupting the surface tension with detergent, and “powering” a boat across the water. Watch the video “Soap Boats.” (Video does not have any narration; music only)
Alternately, you could do this experiment at home. If you wish to do this, to make your first boat:
- Cut out a rectangle from the index card that is 3 inches by 2 inches (the video shows a milk carton being used, but any sort of cardboard, paper, or cardstock will work)
- At one end of the rectangle, cut off the corners so that the front tapers to a point
- In the middle of the back end of the boat cut a notch that is extends ½ inch into the body of the boat and is almost ½ inch wide
- To race the boat:
- Fill a pan with water
- Place the boat on the surface of the water so that it floats (the back of the boat should be at the edge of the pan)
- With a toothpick, dispense a drop of detergent into the notch of the boat
- Note: If the boat stops responding to the detergent, dump out the water, rinse out the tub, and refill with fresh water
3. In your own words, explain why the boat moves forward when detergent is dropped into the water.
BONUS QUESTION
For 2 points of extra credit, provide your solution to the puzzle below:
Rexy the Tyrannosaur fell overboard while sailing one day. Fortunately, Rexy is a pretty good swimmer (in spite of having such short arms), but even so, she needed to find a way to get back onto the ship. The ship’s captain threw a rope down, but it was too short to reach her. The rope stopped 4 ½ feet above the surface of the water. Rexy can reach up 2 feet above the water’s surface.
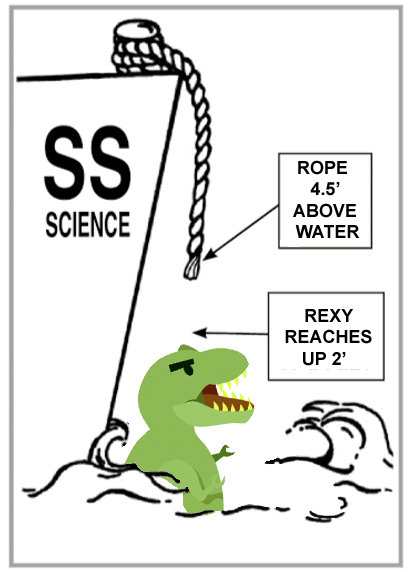
Q: If the tide is rising at 1 foot per half hour, how long will it be before Rexy can reach the end of the rope?
Submit your completed worksheet as requested by your instructor.